Unlocking the Potential of Cryogenic Biobanking: How Multi-Coded Cryostorage Vials Drive Precision and Efficiency in Biomedical Science
In the high-stakes landscape of modern medical research and biotechnology, precision, reliability, and traceability are paramount. One of the cornerstones of effective biobanking and sample management is the ability to store biological specimens under ultra-low temperatures without compromising their integrity. This is where the multi-coded cryostorage vial has emerged as an essential component. With innovations in labeling and tracking technologies, these vials have transformed the efficiency and accuracy of cryogenic storage processes across clinical, academic, and commercial laboratories.
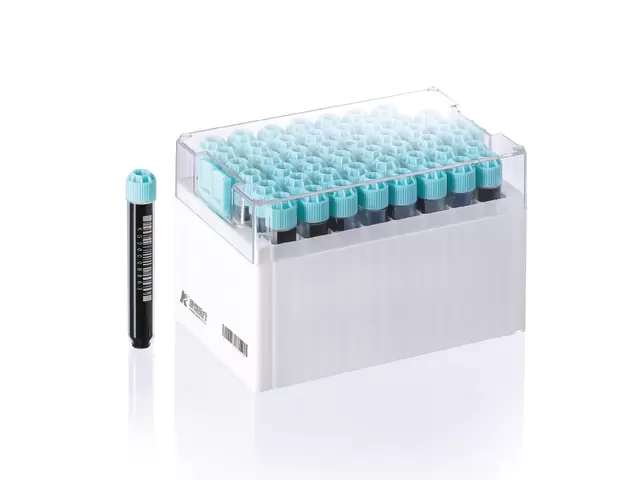
Optimizing Laboratory Operations with Multi-Coded Cryostorage Vials
Enhancing Sample Integrity Through Cryogenic Resilience
Biological materials such as blood, DNA, RNA, cell cultures, and tissue samples are highly sensitive to environmental factors. Even slight deviations in temperature or contamination can result in the degradation of the sample, rendering it unusable for diagnostic or research purposes. Multi-coded cryostorage vials are manufactured using materials such as medical-grade polypropylene that can withstand cryogenic temperatures as low as -196°C without cracking, deforming, or losing their sealing ability. These vials are rigorously tested to ensure they can endure repeated freeze-thaw cycles, which are common in research environments.
Moreover, the sealing mechanisms employed in these vials—including screw caps with silicone O-rings or double-seal designs—provide airtight and leak-proof enclosures. This prevents not only sample loss but also cross-contamination, which is critical when working with hazardous or irreplaceable biological specimens. By preserving the integrity of samples over long durations, multi-coded cryostorage vials enable researchers to maintain consistent and reproducible experimental conditions, which is fundamental to scientific discovery.
Improving Safety and Risk Management
Laboratory safety is an indispensable part of cryogenic storage workflows. The extreme temperatures involved in cryostorage environments pose unique challenges, including risks of physical injury from direct contact with ultra-cold surfaces and exposure to volatile substances. Multi-coded cryostorage vials mitigate these risks through design innovations. Their ergonomic construction facilitates safe handling with cryogenic gloves, while color-coded caps and tactile labeling enhance visual and physical identification in low-visibility storage conditions.
Additionally, the inclusion of non-contact identification technologies like RFID allows for vial recognition without opening freezers, significantly reducing unnecessary exposure to hazardous conditions. By incorporating these features, multi-coded cryostorage vials not only protect valuable biological materials but also contribute to the overall safety of laboratory personnel.
Streamlining Efficiency and Compliance with Multi-Coded Cryostorage Vials
Optimizing Laboratory Efficiency and Workflow
Time and accuracy are critical in laboratory settings, particularly in large biobanks and genomic research centers handling thousands of samples. The multi-coded cryostorage vial streamlines sample retrieval, identification, and inventory management. With multiple layers of identification—including human-readable labels, color codes, 1D/2D barcodes, and RFID chips—laboratories can efficiently implement automated and semi-automated tracking systems.
When integrated with Laboratory Information Management Systems (LIMS), each multi-coded cryostorage vial becomes a node in a highly organized data network. Sample status, location, history, and experimental metadata can be instantly retrieved by scanning the vial, ensuring traceability from sample intake through every step of analysis. This digital integration not only accelerates laboratory workflows but also enhances data quality by reducing transcription errors and manual input.
Supporting Regulatory Compliance and Quality Assurance
Regulatory frameworks governing biomedical research and clinical diagnostics require meticulous documentation and traceability. Multi-coded cryostorage vials support these requirements by offering redundant identification systems that ensure data remains accessible even if one coding method fails. This is vital for compliance with guidelines from institutions such as the FDA, EMA, CAP, and ISO standards.
Each vial can be linked to a digital audit trail, capturing critical information such as collection date, processing protocol, storage conditions, chain of custody, and usage records. This comprehensive documentation is invaluable during internal audits, external inspections, or when submitting data for regulatory approval. Furthermore, batch-specific traceability enhances quality assurance processes by enabling the identification and removal of compromised samples without disrupting the entire storage ecosystem.
The multi-coded cryostorage vial is more than just a container; it is a sophisticated tool that underpins the infrastructure of modern biomedical research and healthcare delivery. From enhancing sample integrity to supporting regulatory compliance and enabling large-scale data integration, these vials play a pivotal role in the advancement of science. As laboratories continue to evolve toward automation, digitization, and personalized care models, the multi-coded cryostorage vial will remain an indispensable asset in ensuring quality, traceability, and efficiency in cryogenic sample management.